



Theme
Nuclear Medicine
INSTITUTION
Cyclotron & Radiopharmaceuticals Department,
King Faisal Specialist Hospital and Research Center,
Riyadh, Saudi Arabia.
Molecular markers for angiogenesis such as (VEGFRs) and the integrins have become the logical targets for investigation. The receptors are known to bind peptides containing the arginine, glycine, and aspartic (RGD) amino acids sequence. These small peptides have been investigated extensively as drugs. Additionally the radiolabeled (F-18, Tc-99m, In-111, and Cu-64) analogs are being investigated as tracers for noninvasive measurement of angiogenesis. These agents have shown promise in both animals and human patients.
The growth of new blood vessels (angiogenesis) sustains tumor spread and growth or metastasizes by supplying oxygen and nutrients. Angiogenesis has been identified as a target site for therapeutic intervention because of its important role in tumor growth, metastasis, and inflammatory diseases. More than 20 angiogenic sites in the extracellular matrix protein laminin-1 have been identified and its crucial role was studied extensively.
We need to develop imaging methods that would measure the actual process at the molecular level. Thus, the objectives of this study were to radiolabeling and in vitro investigation of several peptides that has a potential as an imaging tool for the angiogenesis process. a number of those derivatives, labeled with I-123/125/131 and were compared in vitro. Using human umbilical vein endothelial cells (HUVEC) cell line we evaluate their binding affinity and specificity. The targeted peptide was radioiodinated using the direct electrophilic method via chloramine-T.
Materials and analytical method
All needed reagents and solvents were purchased from Aldrich Chemical Co. or other chemical companies and used with no further purifications unless it’s necessary. Peptide used was purchased from GenScript and used without further purifications. Radioactive Iodine-131 was purchased as sodium-iodide solution and used after dissolving in the buffer of intended reaction. Reaction progress was monitored using both radio-analytical thin-layer chromatography (Radio-TLC) and high pressure liquid chromatographic (Radio-HPLC). HPLC analyses were carried out on semi-preparative Phenomenex C-18 (250 mm x 10 mm). Radiolabeled peptide was detected with the eluting of 0.065%TFA in 100%water (v/v) and 0.05%TFA in 100% MeCN (v/v) at 2 ml/min. Sodium Citrate Buffer (PH=7) were the TLC mobile phases.
Radiolabeling process:
- 20µL of CH-T
- 50µL of peptide (0.1mg dissolve in 50µL DMSO)
- 1 mCi (37 MBq) of the radioactive iodide (Na*I) in 100 µL sodium hydroxide.
- React at RT for 3 min.
- The reaction was stopped by quenching with 50 – 100 µL of 1.0 % sodium metasulfate.
We have radiolabeled the main targeted peptide as a model of the laminin derivatives; with radioactive iodine (123/125/131I). Several labeling technique were used for this investigation and test most of the reaction factors aiming to establish reproducible radiolabeling and analytical procedures. Example of the produced product on radio-TLC, radio-HPLC and initial in vitro study are as follow;
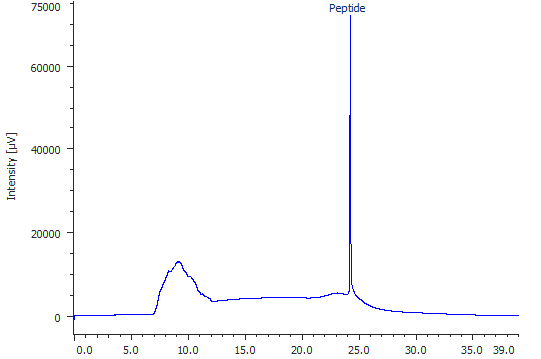
Fig. 1: Mass-HPLC chromatogram of choesen peptide.
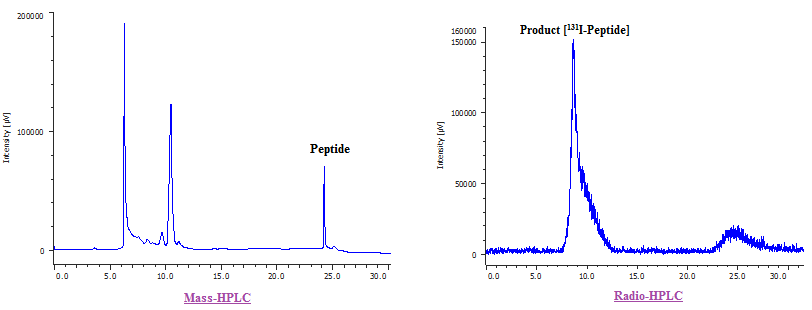
Fig. 2: Mass-HPLC chromatogram of the reaction mixture resulted from the radiolabeling process of Chosen peptide and the Radio-HPLC chromatogram representing the labeled peptide at Rt = 8.6 min with radiochemical purity of 90.33 %.and the RCY=66.44%
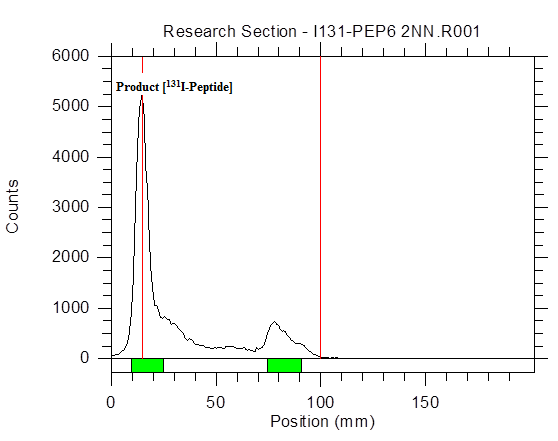
Fig. 3: Radio-TLC, representing the 131I-Peptide (RF=0.00 with 82.63%).
In Vitro study
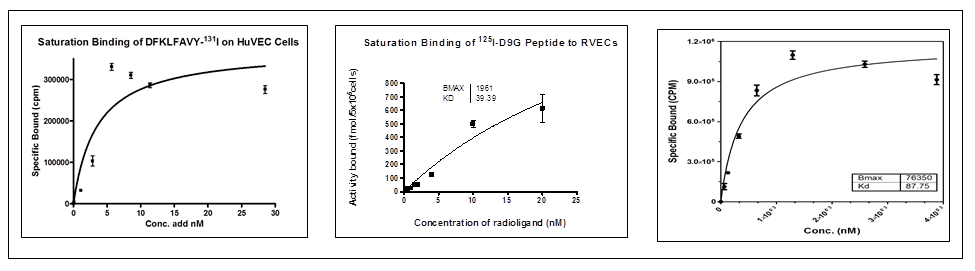
Fig. 4: Examples of the saturation bindings that results of using three different peptides.
- The example peptides were radioiodinated through the direct electrophilic method in high RCY and Purity.
- Results indicates that the chosen peptides that been tested are promising imaging tracers of the angiogenesis biological process. Also it might be useful for treatment management purposes.
This work was supported by King Abdualaziz City for Science & Technology (AT-29-15). We would like to thank Dr. Abdellillah Aboussekhra and Ms. Siti Fauziah Hendrayani for there help in providing the HUVEC cells.
-
Mohammed Al-Qahtani and Yousif Al-Malki (2017), “New labeled 99mTC-HYNIC-short peptide as a potential tumor specific anticancer imaging agent”, J. Label. Compds. Radiopharm., 60, S228.
-
Mohammed Al-Qahtani and Yousif Al-Malki, (2016), “[68Ga]Radiolabelling of oligopeptide that has a pet imaging potential”, presented at the International Conference on Radioanalytical and Nuclear Chemistry (RANC-2016), April 10 – 15, Budapest, Hungary, Book of Abstracts, P 208.
-
Yousif Al-Malki and Mohammed Al-Qahtani (2014), “Evaluating radiolabeled peptides that has a potential as angiogenesis imaging radiotracers”, 2013 KFSH&RC Annual Research Report, April 16-17.
-
Al-Qahtani, Mohammed, Al-Malki, Yousif, (2013) “Radiolabeling of DFKLFAVY peptide as a targeted imaging radiotracer for angiogenesis”, J. Label Compd Radiopharm; 56: S357.
-
Al-Qahtani, Mohammed, Al-Malki, Yousif, (2012) “Radiolabeling short peptides that have a potential as imaging radiotracers” the seventh International Symposium on Radiohalogens (7ISR), 15-19 September 2012; Whistler, B.C., Canada., Abstract book.
-
Al-Qahtani, Mohammed, Al-Khyat, Zenat, (2011) “Radiolabeling and In vitro evaluation of laminin derivatives that blocks angiogenesis and tumor growth”J. Label Compd Radiopharm; 54: S209.
-
Zenat Al-Khyat and Mohammed Al-Qahtani, (2011) “radiosynthesis and in vitro evaluation of laminin derivatives as targeted imaging tracers”, 2010 KFSH&RC Annual Research Report, April 25-26.
-
Mohammed H. Al-Qahtani and John K. Amartey,(2009) “Preliminary Radiolabeling and Evaluation of laminin-1 derived peptide antagonist”, Nucl. Med. Commun., 30; 5 May 2009, P 405
 Send Email
Send Email